Product Description
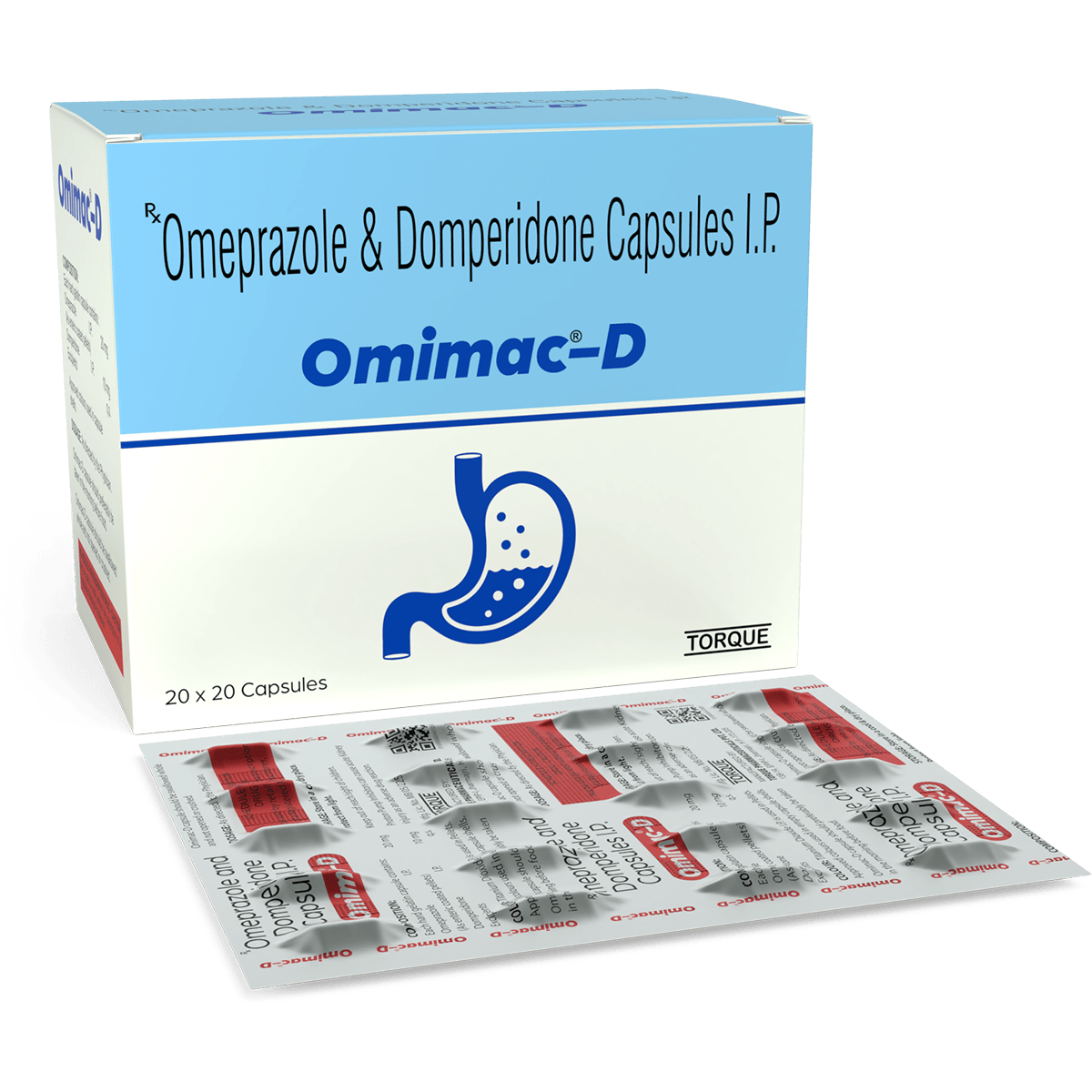
Description: Omimac-D capsule is a combination of proton pump inhibitor (PPI) with anti-emetic medication that reduces stomach acid production, commonly used to treat conditions such as acid reflux, gastroesophageal reflux disease (GERD), and stomach ulcers.
Pharmaceutical Dosage Form: Capsule
Route of Administration: Oral
Composition: Each hard gelatin capsule contains:
Omeprazole (As enteric coated pellets) I.P. 20mg
Domperidone I.P. 10mg
Excipients q.s.
Mechanism of Action: Omeprazole is a proton pump inhibitor, works by inhibiting the proton pump in the stomach lining, specifically the H+/K+ ATPase enzyme. This enzyme is responsible for the final step in the production of stomach acid. By blocking the activity of this enzyme, omeprazole reduces the secretion of gastric acid into the stomach. Domperidoneis a dopamine receptor antagonist used as a peristaltic stimulant and anti-emetic agent for dyspepsia, indigestion, epigastric pain, nausea, and vomiting. It provides relief from nausea by blocking receptors at the chemoreceptor trigger zone. Domperidone is a prokinetic which works on the upper digestive tract to increase the movement of the stomach and intestines, allowing the food to move more easily through the stomach.
Indications: Omimac-D is used to treat indigestion, heartburn, epigastric pain, peptic ulcers, gastro-esophageal reflux disease (GERD), and Zollinger-Ellison syndrome.
Dosage: As prescribed by the physician.
Storage: Store below 250 Protect from light & moisture.
Presentation: 20 x 20 capsules
Side effects:
Nausea
Headache
Stomach pain
Constipation
Flatulence
Diarrhea
Dryness in mouth
Contraindications: Omimac-D capsule is contraindicated in patients with a history of hypersensitivity to the drug or any excipients from the dosage form.
ADR Warning: Proton Pump Inhibitors can cause acute Kidney injury as an adverse drug reaction.